Reliable pull-down assays with MagStrep® Strep-Tactin®XT beads
Knowing which proteins interact with one another is an important step in understanding signaling pathways and specific protein functions, which is key for unraveling mechanisms behind diseases and identifying new targets for drug development. For studying protein-protein interactions, pull-down assays have become an invaluable tool for scientists. In pull-down assays, interactions between two or more proteins are detected, either confirming the predicted protein-protein interaction or discovering new ones. Pull-down approaches represent convenient and low cost in vitro techniques, in which the bait protein is fused to an affinity tag, such as the Twin-Strep-tag®, and immobilized on beads coupled to the corresponding affinity ligand, e.g. Strep-Tactin®XT resin. Afterwards, a protein source containing putative prey is applied. Alternatively, cell lysates containing the tagged bait protein and its potential interaction partners are directly added to the affinity resin. In both cases, the formed bait-prey complexes are eluted with excess chemical or biological reagents releasing the tag from the ligand.
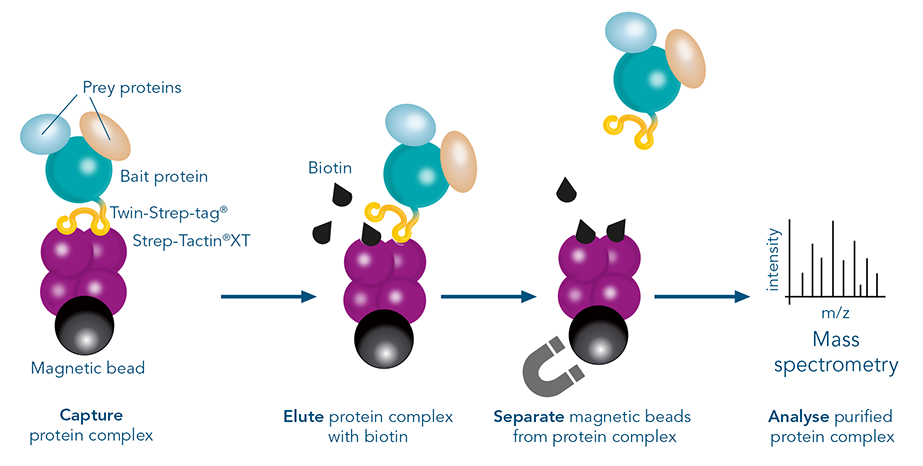
In pull-down assays, interaction partners are “pulled down” along with the tagged protein during purification. Existing protein interactions are preserved during gentle washing steps. In the resulting elution, both the target and its interaction partners are present. Which proteins interacted can be identified through further analysis, for example in mass spectrometry.
MagStrep® Strep-Tactin®XT beads bind to bait proteins tagged with Twin-Strep-tag® with a picomolar affinity, while still maintaining binding reversibility and mild recovery. The specific binding of the Twin-Strep-tag® to Strep-Tactin®XT provides the basis for efficient single-step purification of tagged bait-prey complexes in vitro. Further, the Twin-Strep-tag® is small and chemically inert and thus does neither interfere with proper protein folding and function nor binding interactions in complexes. Mild and adjustable wash conditions ensure that the protein complexes remain intact. Another benefit is the mild elution, as no pH shift or harmful compounds are needed. By simply adding biotin, bait-prey complexes are specifically eluted, reducing co-purification of false positives to almost zero. These characteristics distinguish the Twin-Strep-tag® from other protein tags that are commonly used for pull-down assays such as FLAG-tag, HA-tag or Myc-tag. These epitope tags guarantee efficient immobilization due to an antibody-mediated binding, but the antibodies may also bind non-specific proteins with sequence similarity, leading to false-positive results. In order to achieve a specific elution of epitope tagged proteins, peptides are used in high concentrations, which is very expensive. Alternatively, pH-shift elution is possible, but also elutes unspecific bound proteins. By combining high affinity and high specificity with mild elution conditions, the Twin-Strep-tag® provides a perfect solution for efficient and reliable pull-down assays.

