Bio-layer interferometry
Bio-layer interferometry (BLI) is like SPR a label-free optical biosensing technology for analyzing biomolecular interactions, e.g., antigen-antibody interactions, in real-time and allows quantification of their binding strength and kinetics. For BLI experiments, thin needles, called biosensors, are coupled at the tip with a desired ligand and dipped into the sample to capture the analyte. To measure whether the analyte is bound to the ligand, white light is passed through the biosensor and the reflected light from the tip surface is measured. If the ligand interacts with the analyte, the layer on the surface of the tip is thicker compared to the ligand alone, which leads to a different wavelength that is reflected. The BLI instrument (Octet® or Gator®) measures both the reflected light from the ligand alone and the ligand-analyte complex. The wavelength shift between both reflection patterns creates an interference pattern, which is subsequently used for calculation of binding strength and kinetics.
But what is the best way to bind the ligand to the surface of the biosensor? In principle, the ligand can be bound directly via chemical coupling methods like EDC/s-NHS coupling. But this needs time to find the optimal reaction conditions and can reduce the activity of the ligand. In addition, the orientation of the ligand can vary, which can reduce the accessibility for the analyte and can lead to inconsistent measurement results between the biosensors.
These disadvantages can be prevented if an indirect binding via the Strep-tag® technology is used. Instead of the ligand, Strep-Tactin®XT is first immobilized on the biosensor and the ligand tagged with the Twin-Strep-tag® is captured afterwards by it. Since the tag is at a defined position, all proteins (ligand) captured by Strep-Tactin®XT will be presented in the same orientation. Furthermore, the pM affinity of Strep-Tactin®XT for the Twin-Strep-tag® allows specific capture of Twin-Strep-tagged fusion proteins from complex samples, e.g., culture supernatants, leading to reliable and robust data. All reagents necessary for fast and simple coating of Strep-Tactin®XT biosensors are provided in our Strep-Tactin®XT BLI Coupling Kit and ready-to-use Strep-Tactin®XT biosensors are provided by Gator®.
This application note demonstrates how the Strep-Tactin®XT technology can be used in combination with BLI and SPR analytic devices.
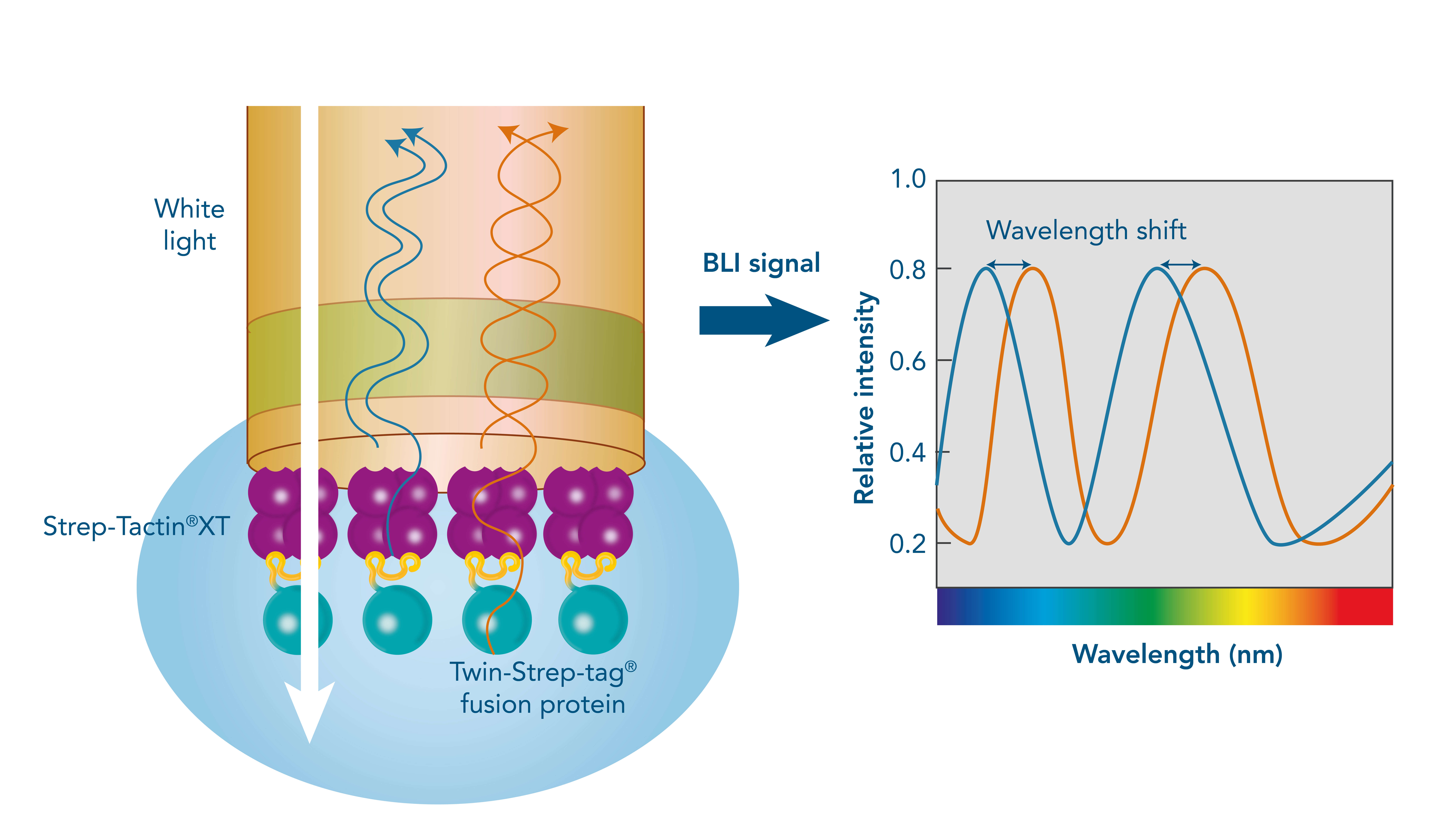
Bio-layer interferometry (BLI) in combination with Strep-Tactin®XT for uniform and specific capture of Twin-Strep-tag® fusion proteins.
Quantification and kinetic analysis of Twin-Strep-tag® fusion proteins
Twin-Strep-tag® is widely utilized for purifying recombinant proteins in fields such as structural biology, enzyme engineering, and protein characterization. Its highly specific binding properties enable efficient purification of target proteins with high purity. The Twin-Strep-tag® is particularly advantageous compared to the His-tag for proteins that need to be purified at physiological pH in order to maintain their functionality.
Gator® Strep-Tactin®XT biosensors
Gator® Strep-Tactin XT probes are specifically designed to capture proteins tagged with Twin-Strep-tag® (TST). These probes are coated with Strep-Tactin®XT, which binds to TST with picomolar (pM) affinity. The Strep-Tactin®XT probe is particularly useful for the quantitation and kinetic characterization of TST-tagged proteins.
Webinar
This webinar describes how Strep-Tactin®XT can be used for efficient purification of nanobodies produced in mammalian cell lines and how Strep-Tactin®XT biosensors can be used to analyze the nanobodies using BLI.

