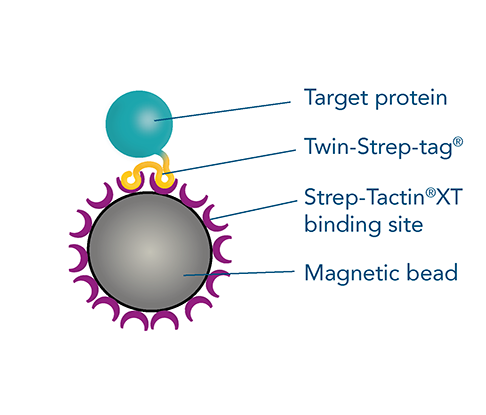
Protein purification with magnetic beads
Magnetic bead-based protein purification is a highly efficient method, particularly for small sample volumes or viscous materials. MagStrep® Strep-Tactin®XT beads are ideal for batch purification, offering easy separation from the supernatant and high specificity, resulting in exceptionally pure proteins. This method is versatile and scalable, suitable for different sample sizes and easily adaptable for automated systems, making it perfect for high-throughput screenings. Coated with the high-affinity ligand, Strep-Tactin®XT, these beads enable specific binding to strep-tagged proteins, providing a simple and customizable workflow for your experimental needs.
It is easy to increase protein yield by adjusting specific parameters when purifying strep-tagged proteins with magnetic beads. Discover these optimization steps below, including protein size, incubation time, bead volume, and protein concentration.
Pull-down assays with magnetic beads
Understanding protein-protein interactions is crucial for studying protein structure and function. Pull-down assays are essential techniques for detecting these interactions, either confirming predicted interactions or discovering new ones. The pull-down method is cost-effective and convenient, using a bait protein fused with an affinity tag, such as the Twin-Strep-tag®, and immobilized on magnetic beads coupled to the corresponding ligand, Strep-Tactin®XT.
Find out more about MagStrep® Strep-Tactin®XT beads, that offer low pM binding affinity with Twin-Strep-tagged bait proteins, ensuring efficient single-step purification of complexes.
From early screening to large scale protein production
Magnetic bead-based purification is highly scalable, making it suitable for processing a wide range of sample volumes. The process can be adapted to different sample sizes, from small-scale high-throughput screenings to large-scale protein production, depending on the application.
Learn more about the flexible scaling of protein purification with magnetic beads in this application note: From Early Screening to Large-Scale Production.
Optimization of magnetic bead-based protein purification
Form |
5% suspension |
| Binding capacity | 42.5 mg/ml (0.85 nmol/µl of a 50 kDa protein) |
| Matrix | 6% agarose, crosslinked, spherical magnetic beads |
| Bead diameter | 30 µm average |
| Specificity | Strep-tag®II and Twin-Strep-tag® |
Optimization parameters
IBA’s protocol for MagStrep® Strep-Tactin®XT beads describe the basic steps for magnetic bead protein purification. Depending on your experimental conditions, it's possible to optimize the purification protocol and increase protein yield by adjusting specific parameters. The following tips and data below will help you optimize magnetic bead protein purification by adjusting bead volume, incubation time, and protein concentration.
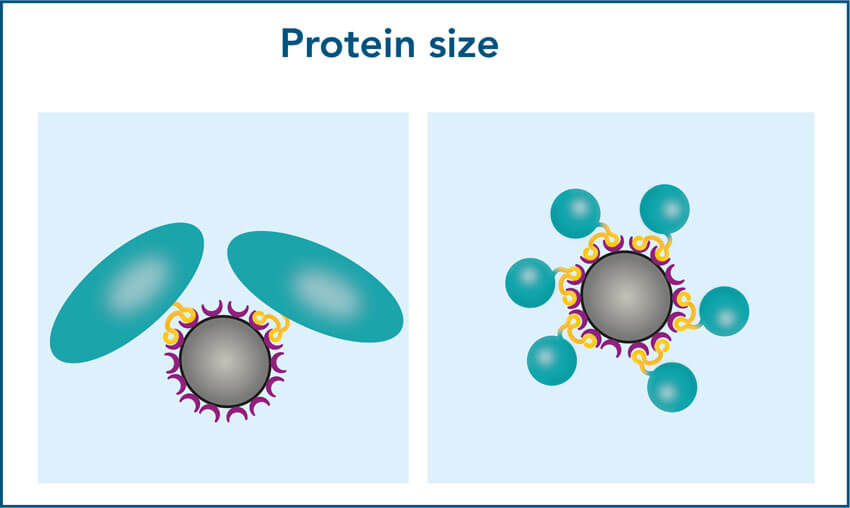
1. Protein size
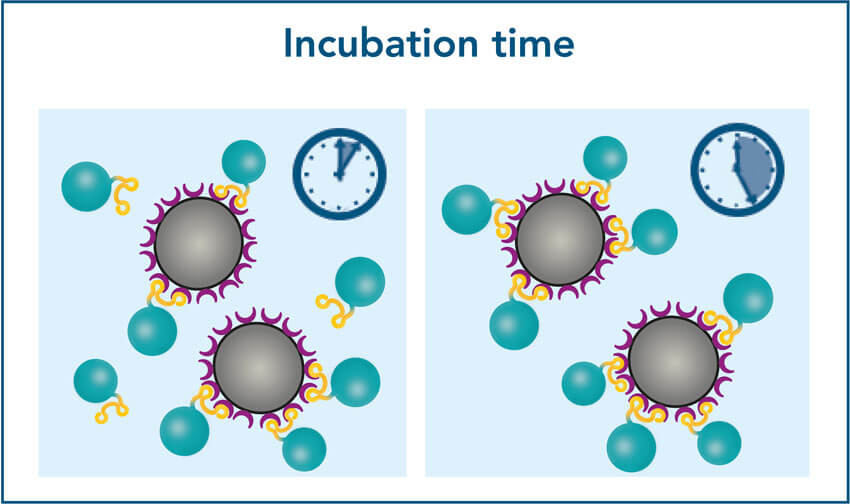
2. Incubation time
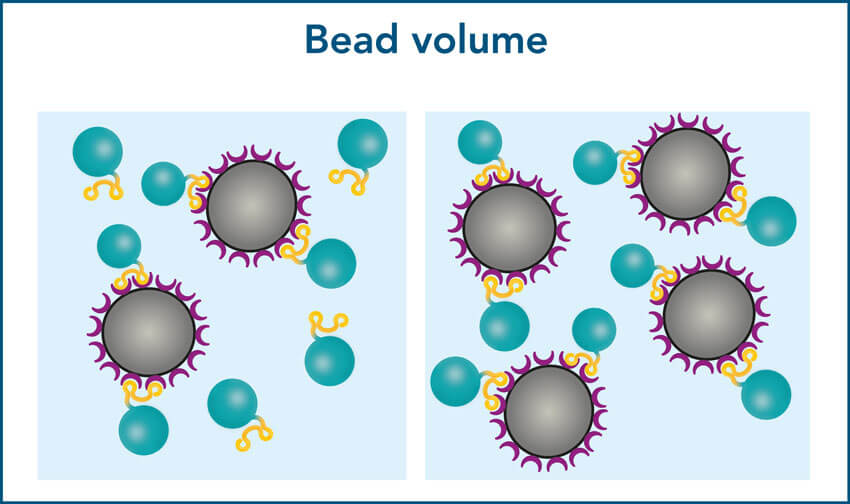
1. Required bead volume depends on the protein size
When working with small proteins, more molecules will bind per bead compared to large proteins. Due to their bigger surface area, large proteins require more space to bind. As a result, the binding capacity may noticeably decline for proteins >90 kDa, which can lead to a lower yield.
To ensure a high yield for large proteins, increase the bead volume to provide a sufficient binding surface. The optimal bead volume for your specific protein can be determined by titration.
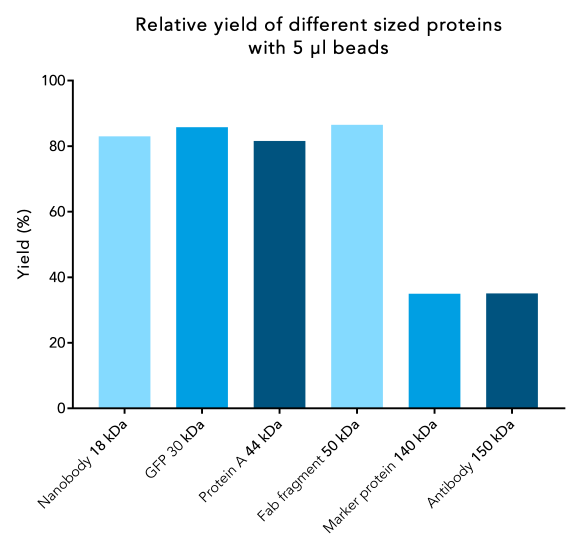
Figure 1: Protein yield decreases for large proteins. 250 µl samples of Twin-Strep-tag® fusion proteins of different sizes with a starting protein concentration of 1.7 nmol/µl were incubated with 5 µl beads. Protein purification was carried out according to IBA’s standard protocol. After elution, the total protein content was compared to the protein content at the beginning.
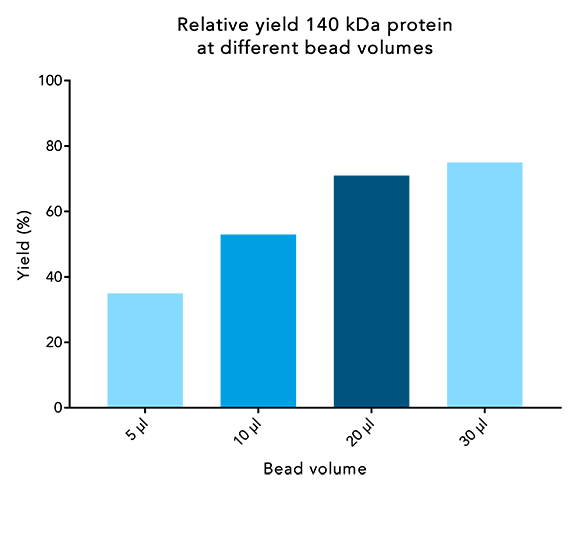
Figure 2: Protein yield of large proteins increases when higher bead volumes are used. 250 µl samples of a 140 kDa protein at 1.7 nmol/µl were incubated with different bead volumes for 10 minutes. Afterwards, the purification was performed according to IBA’s standard protocol. Protein content of the elution was compared to the starting protein content.
2. Increased bead volumes reduce the incubation time
To achieve a higher protein yield in a shorter incubation time, add an excess of beads in relation to the amount of protein and binding capacity of the beads (0.85 nmol/µl beads or 42.5 µg/µl of a 50 kDa protein). Best yield in relation to the bead volume used is reached if 5x more beads are added in relation to the maximum binding capacity. For example, if the total amount of protein is 85 μg, which theoretically could be bound by 2 μl of beads, add 5x 2 μl = 10 μl beads and incubate for 10 minutes to achieve the best yield.
The maximum yield may also be reached when using a lower bead volume, but the incubation time may be noticeably longer.
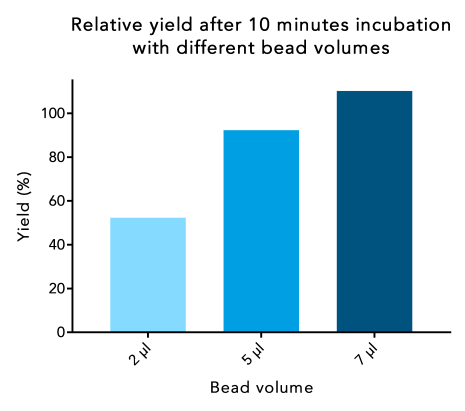
Figure 3: When a higher bead volume is used, protein yield can be increased at a short incubation time. 250 µl samples of a 30 kDa Twin-Strep-tag® fusion protein were incubated with different bead volumes for the same incubation time. Protein content of the elution was compared to the protein content at the start.
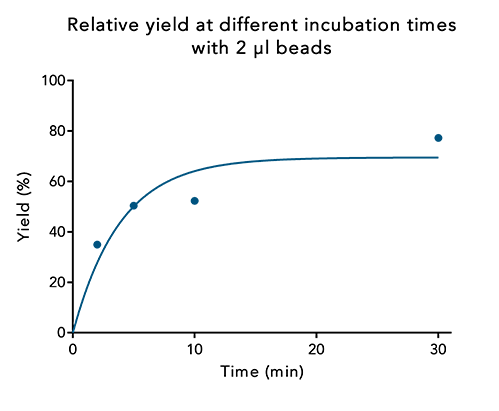
Figure 4: At a low bead volume, protein yield can be increased by incubating for a longer time period. 250 µl samples of a 30 kDa Twin-Strep-tag® fusion protein were incubated with the same bead volume for different time periods. Protein content of the elution was compared to the protein content at the start.
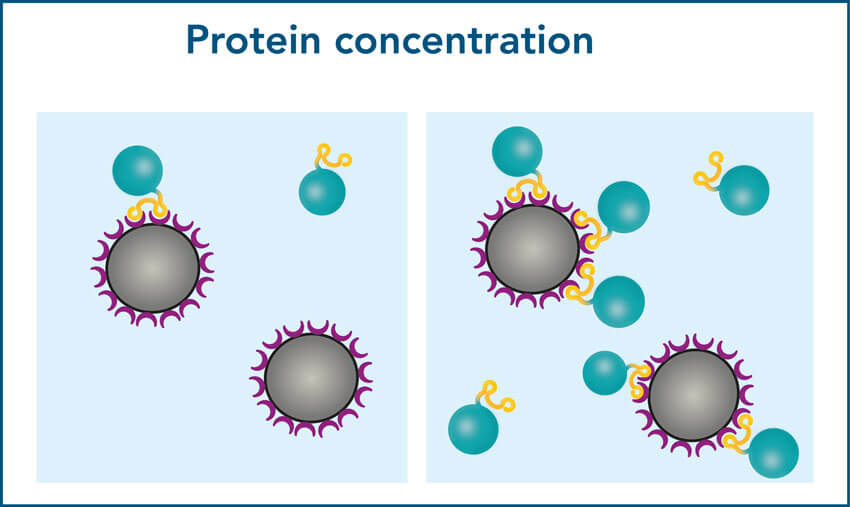
3. Sample protein concentration and bead volume must be balanced
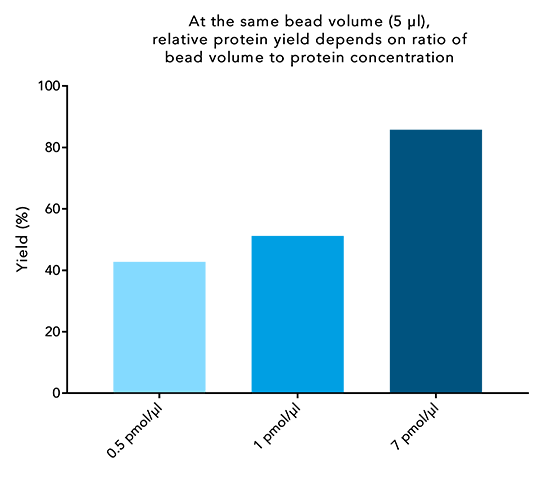
Figure 5: Higher protein concentrations lead to higher protein yield. 250 µl of different concentrated samples of a 30 kDa Twin-Strep-tag® fusion protein were incubated with 5 µl beads for 10 minutes. IBA’s standard protocol was carried out afterwards and the final protein content of the elution was compared to the protein content at the start.
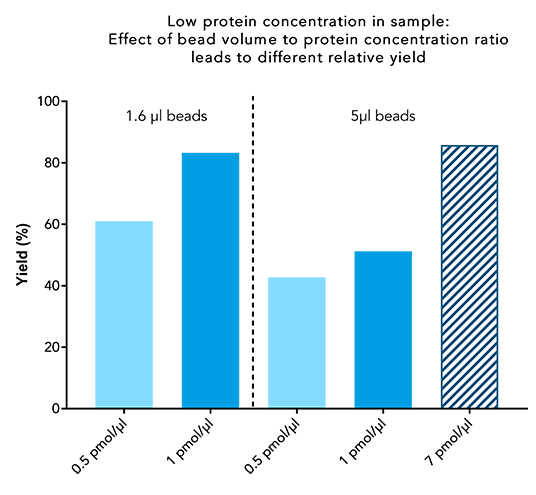
Figure 6: A high bead volume does not lead to a high yield when working with low protein concentrations. 250 µl sample containing 0.5 or 1 pmol/µl of a 30 kDa Twin-Strep-tag® fusion protein, were incubated with 1.6 or 5 µl beads for 10 minutes. IBA’s standard protocol was carried out afterwards, and the final protein content of the elution was compared to the protein content at the start.
FAQ about magnetic bead purification
Application example
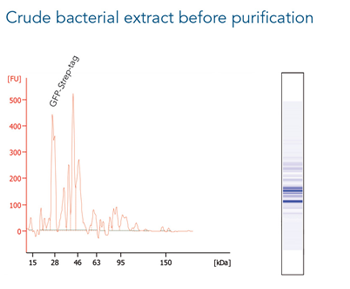
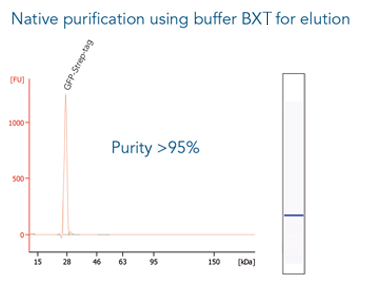
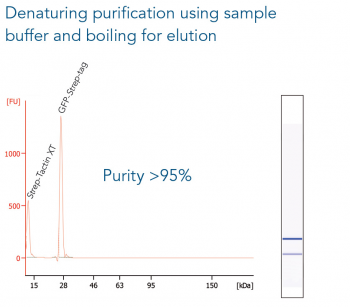
GFP C-terminally tagged with Strep-tag®II (28 kDa) was expressed in E. coli (A) and purified with MagStrep® Strep-Tactin®XT beads (MagStrep "type3" XT beads). For separation of magnetic beads from residual solution, the Magnetic Separator was used. Before elution, the sample was split and the target protein was eluted either by application of 1x Buffer BXT containing biotin or by boiling (C, 5 min at 95 °C). Due to boiling, the Beat´s agarose melts and Strep-Tactin®XT is released, leading to a further peak at 14 kDa. Protein purification results were analyzed with the Agilent Bioanalyzer 2100 system. The example shows the specific binding properties of MagStrep® Strep-Tactin®XT beads (MagStrep "type3" XT beads) and the high purity that can thereby be observed.