Application Examples
Browse through our application examples demonstrating, how IBA products were used in the various life science research projects conducted by both our customers and our in-house team of scientists and which results were obtained.
Cloning & Transfection
MEXi - Mammalian Expression System
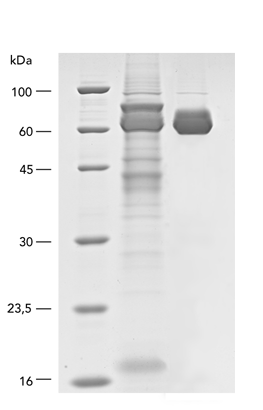
Type: metalloprotein, hydrolase
Yield : 143 mg/l.
Secreted Alkaline Phosphatase (SEAP) was fused with a C-terminal Twin-Strep-tag® and the BM40 secretion signal via cloning into pDSG-IBA102. MEXi 293E cells (1.5 x 106 cells/ml) were transfected in MEXi-TM transfection medium (17 ml) with polyethylenimine (PEI, 25 kDa). Afterwards, the cell culture was incubated for 4 hours (37 °C, 5% CO2, 125 rpm) and then diluted with MEXi-CM culture medium to reach a cell density of 0.75 x 106 cells/ml. The cells were kept at 37 °C, 5% CO2, and 125 rpm for 7 days in order to obtain high protein yields. For purification, the cells were pelleted and the supernatant, containing the SEAP protein, was harvested. Free Biotin was blocked by BioLock containing avidin. The SEAP protein was finally purified using a gravity flow Strep-Tactin® Superflow® high capacity column.
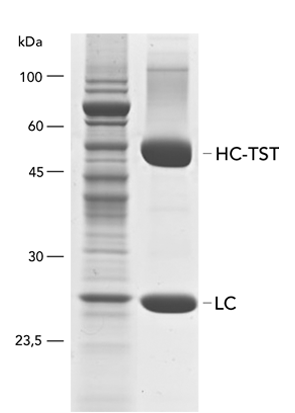
Type: antibody
Yield: 96 mg/l (represented by heavy (HC) and light chain (LC))
The monoclonal rat antibody (mAb) was cloned into the pDSG-IBA102 vector in order to fuse the heavy chain (HC) C-terminally with the Twin-Strep-tag® and the BM40 secretion signal. The transfection of MEXi 293E cells was performed in MEXi-TM transfection medium (250 ml) with polyethylenimine (PEI, 25 kDa). The cells were incubated for 4 hours (37 °C, 5% CO2, 125 rpm) and when a cell density of 3 x 106 cells/ml was reached, 250ml MEXi-CM culture medium was added. Afterwards, the culture was shifted to 32 °C and incubated until day 10.
In order to divide the cells from the supernatant, the cell suspension was centrifuged according to the MEXi manual. Free biotin was blocked by BioLock containing avidin. The supernatant was used for protein purification via a gravity flow Strep-Tactin® Superflow® high capacity column. WET FRED was used to facilitate loading of the large supernatant volume onto the column.
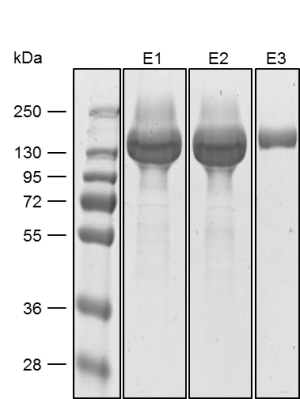
Yield: 318 mg/l
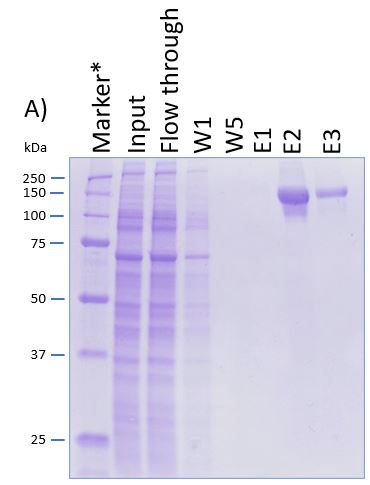
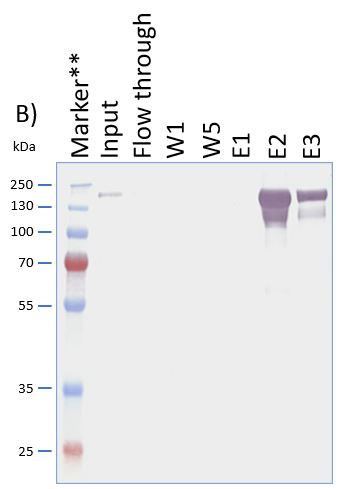
The coding sequence of the customer protein was cloned into pDSG-IBA102 leading to a recombinant protein with C-terminal Twin-Strep-tag® and BM40 secretion signal. 1050 ml of MEXi-TM was inoculated with MEXi 293E cells. Subsequently, plasmid DNA was added followed by addition of 25 kDa linear PEI. The cells were incubated for 4 hours in MEXi-TM medium at 37 °C, 5% CO2, and 125 rpm in an orbital shaking incubator. Cells were diluted to 7.5 x 105 cells/ml by addition of one volume MEXi-CM and kept at 37 °C for 7 days. Afterwards, cells were pelleted and the supernatant containing the customer protein was harvested. The customer protein was finally purified using Strep-Tactin®XT Superflow®. The figure shows the elution fractions (E1-E3). A dilution of 1:10 was prepared for E1 and E2 for SDS-PAGE analysis.
StarGate Cloning
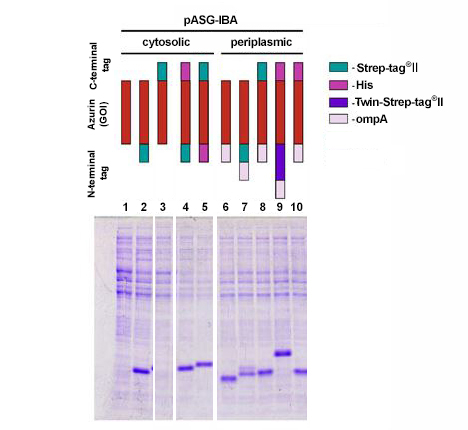
The coding sequence of azurin, a 14 kDa bacterial protein, was cloned into 10 different pASG-IBA vectors and expressed in E. coli. Comparable amounts of E. coli cells were harvested 3 hours after induction with anhydrotetracycline. Samples were lysed by boiling for 5 min at 95 °C with gel loading buffer and analyzed by SDS-PAGE. Subsequently, the gel was stained with Coomassie. Periplasmic secretion by means of ompA led in all cases to accumulation of comparable amounts of the protein of interest (lanes 6-10). This could be expected as azurin is also secreted in its authentic host P. aeruginosa. In case of cytosolic expression, however, interesting aspects became obvious, since it was not anticipated that cytosolic expression of azurin is possible at all. Expression was enabled by fusion of an N-terminal affinity tag (lanes 2, 4, and 5), while N-terminal untagged variants did not result in expression (lane 1 and 3). The examples show that initial screening for expression conditions may be worthwhile since different constructs can lead to various results depending on the target protein properties.
Protein Affinity Chromatography
Protein purification with Strep-Tactin®XT
For protein purification of Strep-tag®II and Twin-Strep-tag® proteins with Strep-Tactin®XT IBA Lifesciences offers MagStrep® Strep-Tactin®XT beads (MagStrep "type3" XT beads), 4Flow®, and pre-packed Spin Columns. As example, the purification of different target proteins with Strep-Tactin®XT 4Flow® and MagStrep® Strep-Tactin®XT beads (MagStrep "type3" XT beads) is shown.
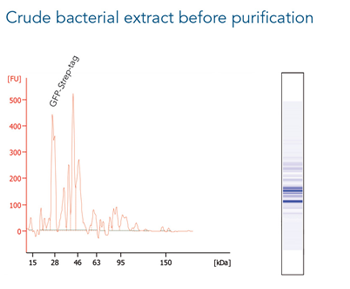
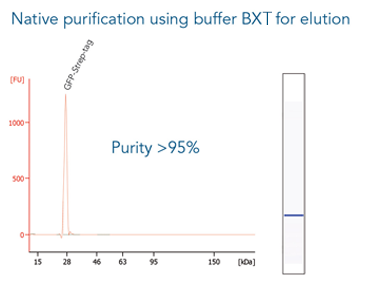
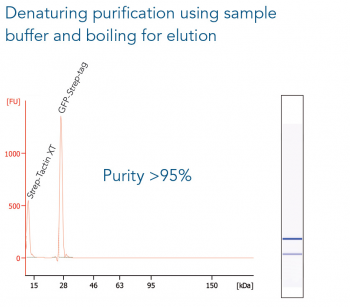
GFP C-terminally tagged with Strep-tag®II (28 kDa) was expressed in E. coli (A) and purified with MagStrep® Strep-Tactin®XT beads (MagStrep "type3" XT beads). For separation of magnetic beads from residual solution, the Magnetic Separator was used. Before elution, the sample was split and the target protein was eluted either by application of 1x Buffer BXT containing biotin or by boiling (C, 5 min at 95 °C). Due to boiling, the Beat´s agarose melts and Strep-Tactin®XT is released, leading to a further peak at 14 kDa. Protein purification results were analyzed with the Agilent Bioanalyzer 2100 system. The example shows the specific binding properties of MagStrep® Strep-Tactin®XT beads (MagStrep "type3" XT beads) and the high purity that can thereby be observed.
Protein purification with Strep-Tactin®
IBA offers MacroPrep®, Superflow®, and Sepharose® coupled with Strep-Tactin® for protein purification of Strep-tag®II and Twin-Strep-tag® proteins. As example, the purification of different target proteins with Strep-Tactin® Sepharose® or Strep-Tactin® Superflow® is shown.
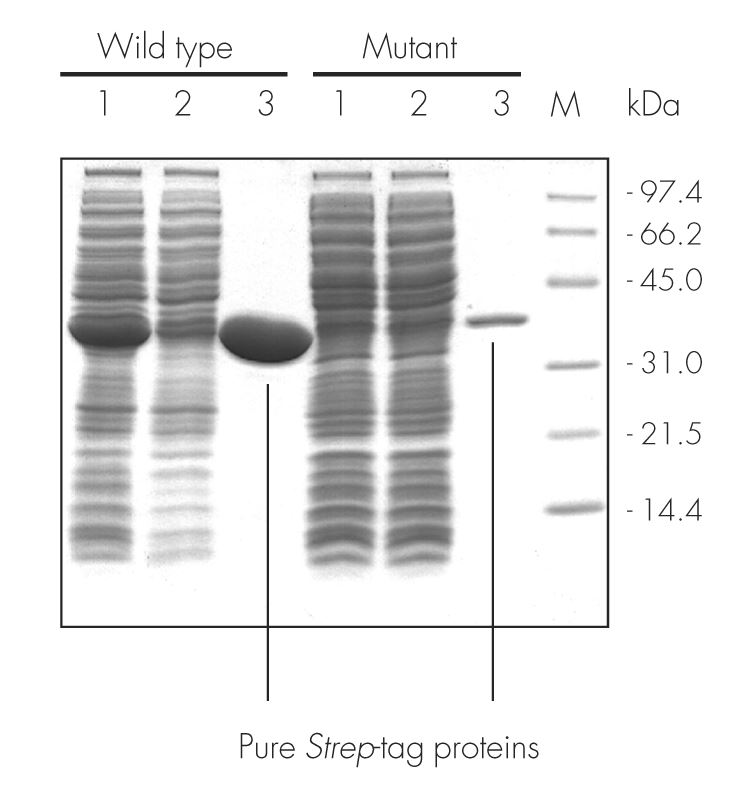
The wild type sequence and mutant variant of an enzyme were cloned into pASK-IBA3, leading to proteins localized to the cytoplasm and C-terminally tagged with the Strep-tag®II. After expression in E. coli and cell lysis, the proteins were purified with Strep-Tactin® Sepharose® gravity flow columns under physiological conditions (100 mM Tris-Cl, pH 8.0). Purification results were analyzed by SDS-PAGE. A molecular weight marker (M), cell lysate (lane 1), flow through (lane 2), and elution with 2.5 mM desthiobiotin (lane 3) are shown. For both proteins, a high purity was observed.
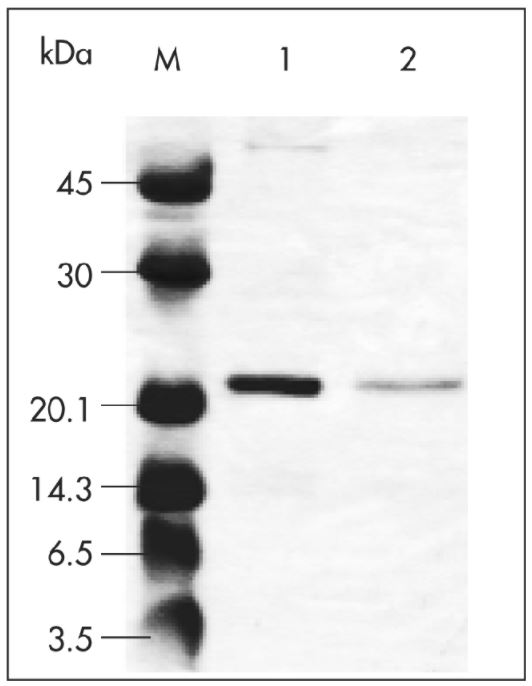
Coding sequences of virus-like particles (VLPs, 4.8 MDa) were cloned into pASK-IBA7 to obtain proteins localized to the cytoplasm and N-terminally tagged with Strep-tag®II. The plasmid was transformed into E. coli and the cell culture (1 l) was induced at OD600 of 0.6 by addition of anhydrotetracycline (AHT). Protein expression was performed at 37 °C for 3 h at 200 rpm. Afterwards, cells were harvested, resuspended with 20 ml 1x Buffer W (100 mM Tris-Cl, 150 mM NaCl, 1 mM EDTA), and sonicated. The insoluble material was pelleted, and the lysate was applied to a Strep-Tactin® Superflow® FPLC column (1 ml/min flow rate). After washing, the target protein was eluted with 2.5 mM desthiobiotin. Purification results were analyzed by SDS-PAGE. Marker (M) and two different elution fractions of VLPs fused to Strep-tag®II (lane 1 and 2) are shown.
Data kindly provided by L. Stöckl and B. Brandenburg, Robert-Koch-Institute, Berlin. Brandenburg, et al. (2005): A Novel System for Efficient Gene Transfer Into Primary Human Hepatocytes Via Cell-Permeable Hepatitis B Virus–like Particle, HEPATOLOGY (42), 1300-1309.
Resin Comparison
Both Strep-Tactin® and Strep-Tactin®XT resins can be used for purification of Strep-tag®II and Twin-Strep-tag® proteins. However, due to their different properties, an initial comparison can be helpful to find the appropriate resin.
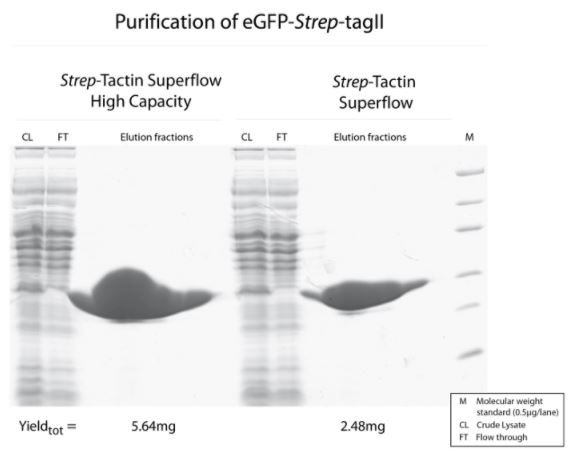
Cell lysate contaning GFP-Twin-Strep-tag was split and either purified with Strep-Tactin® Superflow® or Strep-Tactin® Superflow® high capacity.
Both purifications led to a highly pure protein, but due to the higher density of immobilized Strep-Tactin® on the high-capacity variant, a two-fold higher amount of GFP-Twin-Strep-tag can be purified with Strep-Tactin® Superflow® high capacity compared to Strep-Tactin® Superflow®.
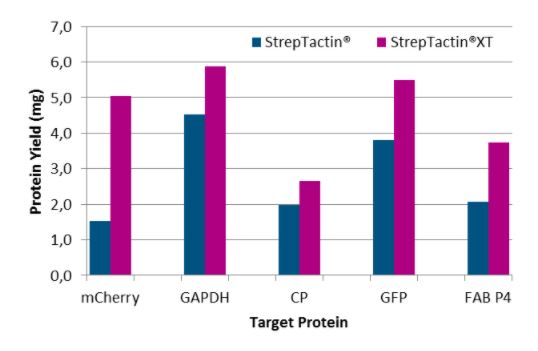
Five different proteins were purified with Strep-Tactin® Superflow® and Strep-Tactin®XT Superflow® to compare the yield with both rein types. Here, we found that Strep-Tactin®XT Superflow® allows the purification of on average a 2-fold higher protein yield than Strep-Tactin® Superflow®.
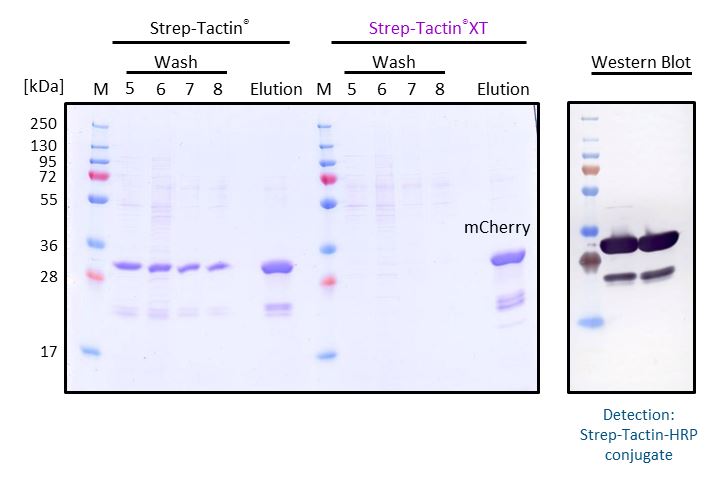
An advantage of Strep-Tactin®XT compared to Strep-Tactin® is that it allows intensive washing without loss in protein yield. To illustrate this, mCherry-Twin-Strep-tag from E. coli supernatant was purified with a gravity flow column either containing 1 ml Strep-Tactin® Superflow® or Strep-Tactin®XT Superflow®. During purification, the columns were washed with 8 column volumes of 1x Buffer W to reach a constant absorption ratio at A260/280 nm. Then, the target protein was eluted with 50 mM biotin. Samples of the last wash fractions (wash 5-8) and the elution fraction were analyzed by SDS-PAGE. In comparison to Strep-Tactin® Superflow®, Strep-Tactin®XT Superflow allows intensive washing without loss of protein yield. The high affinity (pM range) between Strep-Tactin®XT and Twin-Strep-tag® leads to a tighter binding and prevents a premature elution of the target protein during the wash steps. The elution fractions were further analyzed by Western Blot and detected with Strep-Tactin®HRP, showing that the further bands are alternative variants of mCherry-Twin-Strep-tag.
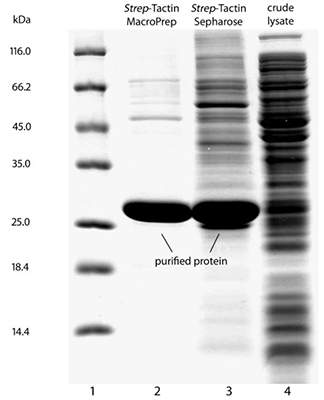
For some recombinant proteins, for example from plants, purification results of Strep-Tactin® MarcoPrep® can differ from those of Strep-Tactin® Sepharose®, due to different resin properties. The SDS-PAGE gel shows the purification of a recombinant protein which exhibits exceptionally high non-specific protein binding. In this case, contaminants were almost completely removed by application of Strep-Tactin® MacroPrep®, whereas the contaminants co-eluted with the recombinant protein after the same purification protocol using Strep-Tactin® Sepharose®. Please note, normally behaving recombinant proteins can be purified in a single step to homogeneity using Strep-Tactin® Sepharose®, however, the example shows that it may be reasonable to change the resin if non-specific protein binding occurs.
Protein Immobilization
Strep-Tactin®XT
Strep-Tactin®XT binds with high affinity Twin-Strep-tag® proteins, making it perfectly suitable for SPR analysis. The examples show the coupling of Strep-Tactin®XT on different SPR chips and possible regeneration conditions for re-use of Strep-Tactin®XT-coated chips.
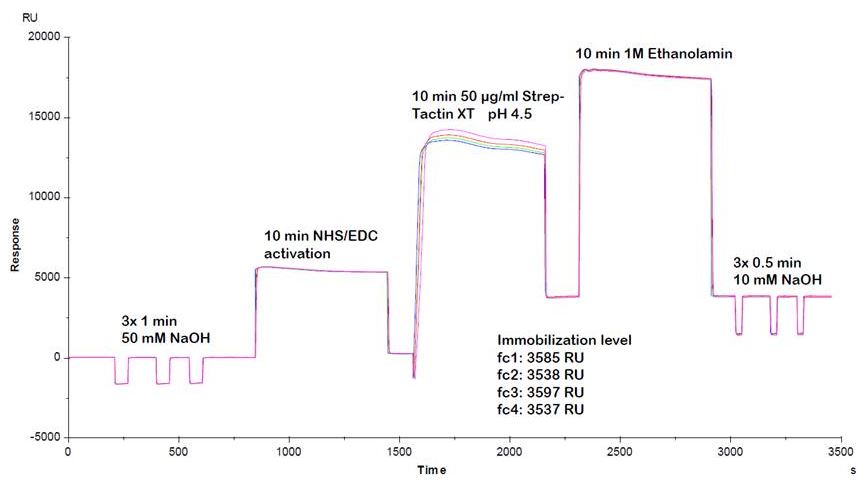
We recommend to use the following conditions for optimal Strep-Tactin®XT coupling:
- 20-50 µg/ml Strep-Tactin®XT
- max. 20 mM acetate, pH 4.5
Using these conditions for Strep-Tactin®XT coupling, efficient and reproducible coupling results can be obtained.
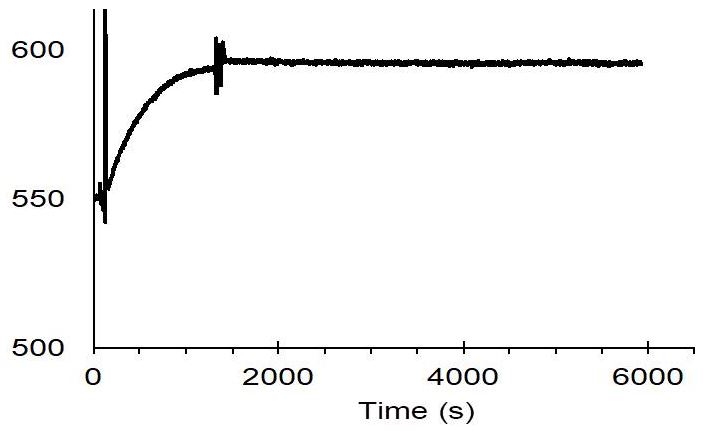
The membrane protein CB2 was fused with Twin-Strep-tag® and then immobilized on a CM4 sensor chip coated with Strep-Tactin®XT.
Used conditions:
- 50 mM Tris pH 7.5, 100 mM NaCl
- 10 % glycerol, 0.1 % DDM, 0.5 % CHAPS, 0.1 % CHS
Data was published in A. Yeliseev et al., Protein Expression and Purification 131 (2017) 109-118
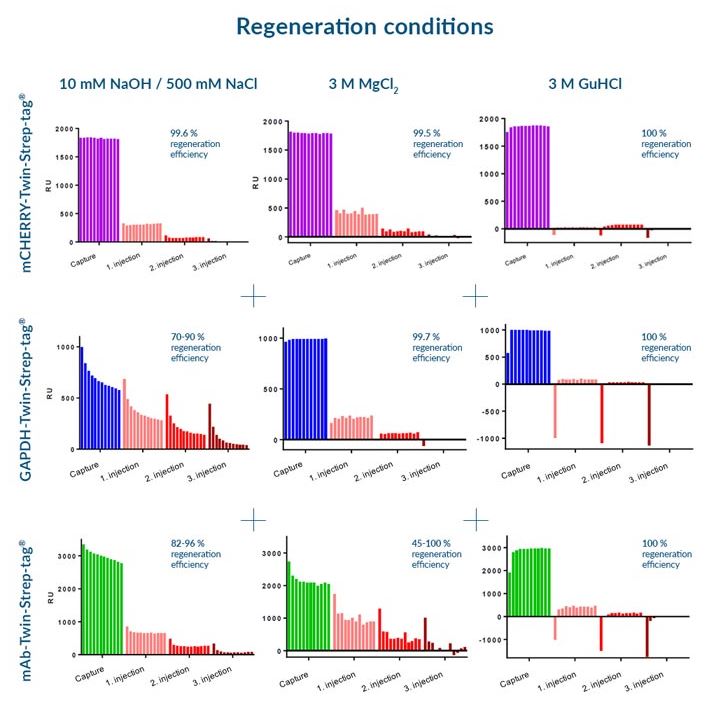
Three different regeneration conditions were tested on Strep-Tactin®XT CM5 sensorchips:
- 10 mM NaOH / 500 mM NaCl
- 3 M MgCl2
- 3 M GuHCl
for three different Twin-Strep-tag® fusion proteins:
- mCherry-Twin-Strep-tag
- GAPDH-Twin-Strep-tag
- mAb-Twin-Strep-tag
We recommend the application of 3 M GuHCl for optimal regeneration conditions, since this buffer led to the best regeneration results for all test proteins. However, depending on the target protein, other buffers are applicable as well.
StrepMAB-Immo
Due to the nearly irreversible binding of Strep-tag®II proteins, StrepMAB-Immo can be used for SPR analysis. The example shows the stable capture of a Strep-tag®II fusion protein on a SPR chip coated with StrepMAB-Immo.
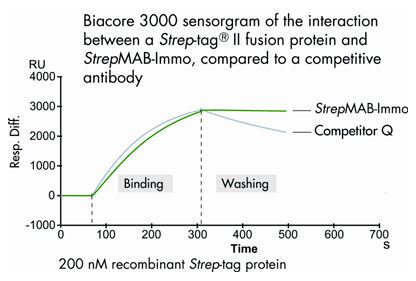
Chips for SPR were coated either with StrepMAB-Immo or with a competitive antibody (Competitor Q). Subsequently, a Strep-tag®II fusion protein was captured, and the binding stability was determined using a Biacore 3000.
During the washing phase, the recombinant Strep-tag® protein of interest remains tightly bound to StrepMAB-Immo, while a significant amount of Strep-tag® protein is washed off using the competitive antibody.
Strep-Tactin® microplates
Strep-Tactin® coated microplate was incubated with different amounts of recombinant E. coli alkaline phosphatase (AP) tagged with the Strep-tag®II. After several washing steps, activity of bound fusion protein was determined by colorimetric reaction.
Strep-Tactin® coated microplate was incubated with recombinant H. pylori urease tagged with Strep-tag® II, followed by three washing cycles. Afterwards, the microplate was incubated with human sera followed by three washing cycles. Third incubation occurred with rabbit anti-human lgG conjugated to horseradish peroxidase (HRP), followed by 3 washing cycles. Amount of bound antibodies from human sera was determined by colorimetric reaction of HRP.
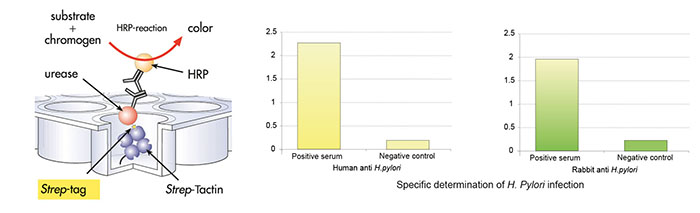
Staining & Detection
Protein detection with Strep-Tactin® Conjugates
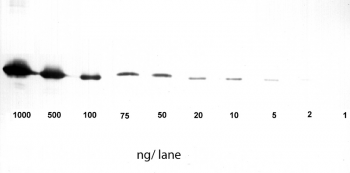
Application: Western Blot
1-1000 ng/lane of GFP C-terminally tagged with Strep-tag®II (28 kDa) were separated by SDS-PAGE and blotted onto a membrane. Detection occurred with Strep-Tactin® HRP.
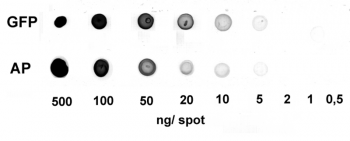
Application: Dot Blot
0.5-500 ng/spot of GFP-Strep-tagII (28 kDa) or alkaline phosphatase C-terminally tagged with Strep-tag®II (monomer 48.5 kDa) were spotted onto a membrane. Detection occurred with Strep-Tactin® HRP.
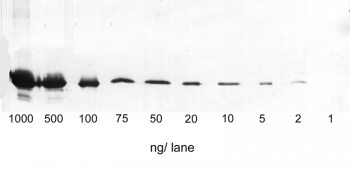
Application: Western Blot
1-1000 ng/lane of GFP C-terminally tagged with Strep-tag®II (28 kDa) were separated by SDS-PAGE and blotted onto a membrane. Detection occurred with Strep-Tactin® AP.
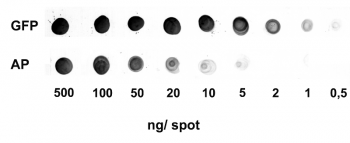
Application: Dot Blot
0.5-500 ng/spot of GFP-Strep-tagII (28 kDa) or alkaline phosphatase C-terminally tagged with Strep-tag®II (monomer 48.5 kDa) were spotted onto a membrane. Detection occurred with Strep-Tactin® AP.
Detection with Strep-tag® Antibodies
StrepMAB-Classic and StrepMAB-Immo can be used for detection of Strep-tag®II or Twin-Strep-tag® proteins on cell surfaces, within the cell, in cell lysates, or in eluates after protein purification. As example, the application for flow cytometry and confocal microscopy is shown.
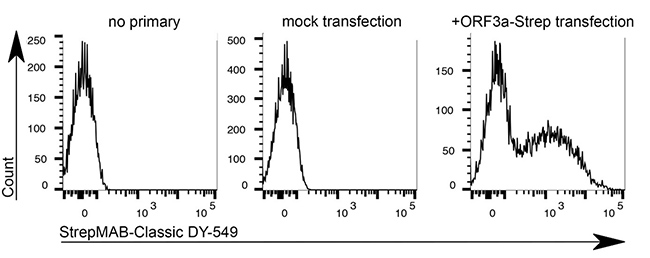
Application: Flow cytometry
Dilution: 1:500
HeLa cells were transiently transfected with the coding sequence of SARS-CoV-2 ORF3a-Twin-Strep-tag. After 48 hours transfection, cells were detached, gently trypsinised and stained with StrepMAB-Classic DY-549 (1:500, 0.5% BSA + 1 mM EDTA, 30 min on ice). Intracellular SARS-CoV-2 ORF3a-Twin-Strep-tag levels were measured by flow cytometry (4 laser Cytoflex S, Beckman Coulter)
Source: Data kindly provided from James Edgar, Department of Pathology at the University of Cambridge.

Application: Confocal microscopy (Immunofluorescence)
Dilution: 1:500
HeLa cells were grown on glass coverslips, fixed (4% PFA/PBS), quenched (15 mM glycine/PBS), permeabilized (0.1% saponin/PBS), blocked (1% BSA, 0.01% saponin in PBS), and stained with StrepMAB-Classic (1:500) to confirm the expression of SARS-CoV-2 ORF3a-Twin-Strep-tag. Anti-mouse 488 was used as secondary antibody. Nuclei were stained with DAPI and the imaging occurred with a LSM700 confocal microscope (63×/1.4 NA oil immersion objective; ZEISS).
Source: Data kindly provided from James Edgar, Department of Pathology at the University of Cambridge.
Fluorescent cell staining for flow cytometry with Strep-Tactin® and Strep-Tactin®XT conjugates
The Strep-tag® technology provides different options for cell staining.
Direct staining of cells expressing a protein with a Strep-tag®II or a Twin-Strep-tag® on the surface
Indirect staining via a cell-binding protein (e.g. a Fab fragment, a MHC molecule or an antigen) that is fused to a Strep-tag®II or a Twin-Strep-tag®
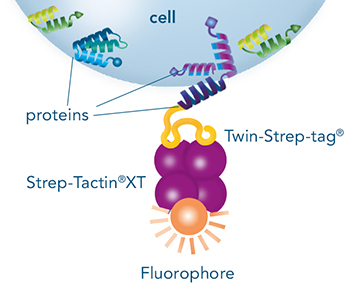
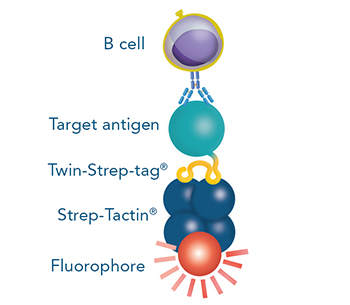
The Strep-tag®II or Twin-Strep-tag® are short peptide sequences that can be expressed on a cell surface without affecting the properties of the cell. Fusing one of the tags to a surface protein of choice enables easy selection of cells that successfully express this protein.
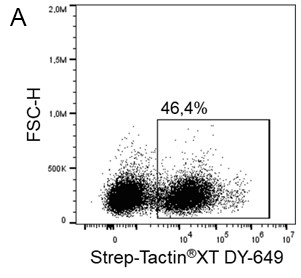
MEXi cells expressing a surface protein fused to a Twin-Strep-tag® were mixed with native MEXi cells at a ratio of approximately 1:1. 1 x 10^6 total cells were stained with Strep-Tactin®XT DY-649 at a dilution of 1:5000* (A) or with Strep-Tactin®XT APC at a dilution of 1:1000* (B). Staining was performed in 100 µl Buffer CI (1x PBS with 1 mM EDTA and 0,5% BSA) for 30 min at 4°C.
*Titration of optimal dilutions might be required for individual experimental setups. In addition, the choice of conjugate depends on factors such as protein density on the cell surface. Due to their brighter staining, PE and APC conjugates can be advantageous for low expressed targets.
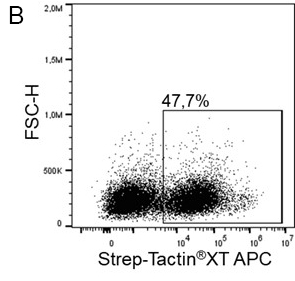
For cytotoxic T cell staining, 200 ng anti-human CD8 Fab fragment (50 kDa) fused to a Twin-Strep-tag® was pre-incubated with 75 ng of Strep-Tactin®XT APC (A) or Strep-Tactin®XT DY-649 (B) for 10 min at 4 °C. The formed complexes were added to 5 x 106 peripheral blood mononuclear cells (PBMCs) and incubated for 20 min at 4 °C in the dark. Afterwards, additional staining antibodies were added. Both fluorescent conjugates achieved clear separation of positive and negative cell populations, demonstrating a flexible choice of fluorophores for highly expressed target proteins such as CD8 on the surface of T cells.
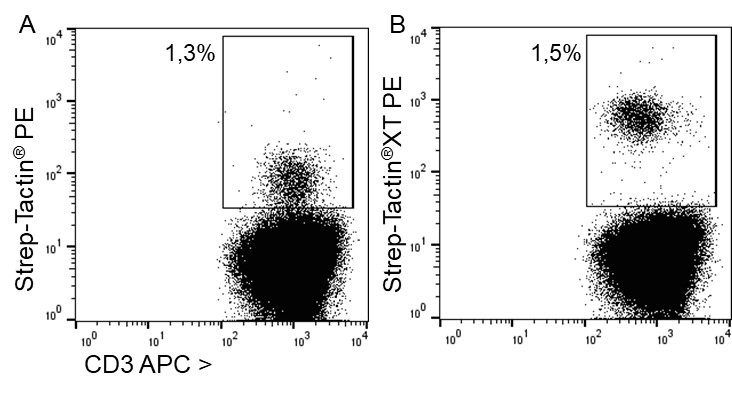
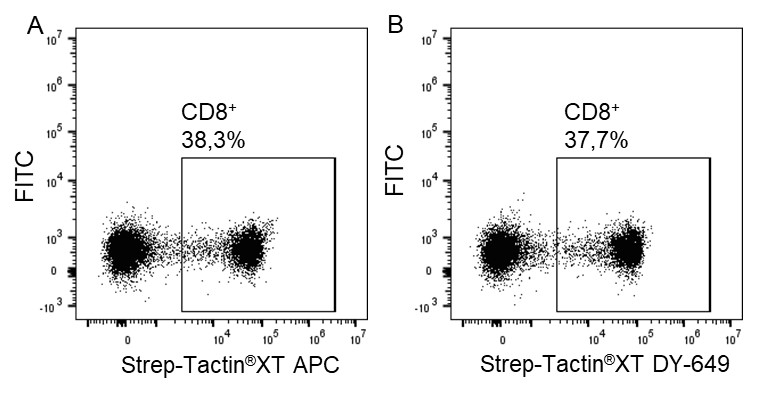
For staining low affinity targets such as T cell receptors, multimerization of the ligand is required to enable stable binding of the detection reagent to the cell. This multimerization can be achieved with Strep-Tactin® or Strep-Tactin®XT PE or APC conjugates.
For antigen specific T cell staining, 200 ng of an MHC class I molecule fused to a Twin-Strep-tag® (50 kDa) refolded with a cytomegalovirus (CMV)-derived peptide was pre-incubated with 75 ng of Strep-Tactin® PE (A) or Strep-Tactin®XT PE (B) for 15 min at 4 °C. 1 x 107 PBMCs in 100 µl buffer were added to the staining complexes and incubated for 20 min at 4 °C. Afterwards, additional staining antibodies were added.
Strep-Tactin®XT PE achieved a brighter staining intensity than Strep-Tactin® PE, indicating that Strep-Tactin®XT PE and APC are the preferred conjugates for low affinity and low expressed targets such as specific T cell receptors. Strep-Tactin®XT DY-649, DY-549 and DY-488 conjugates are not recommended for this approach, since their lesser brightness does not achieve sufficient staining (data not shown).

