Strep-tag® technology for cell isolation
Besides the well-known tools for protein purification and analyses, the Strep-tag® technology also offers methods for cell staining and isolation. The two main approaches are:
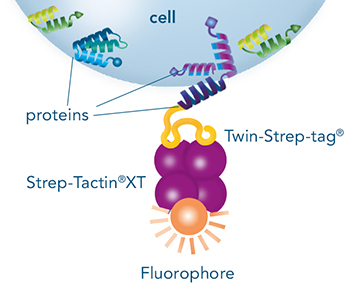
Direct staining and/or isolation of cells that express a surface protein fused to Strep-tag®II or Twin-Strep-tag®.
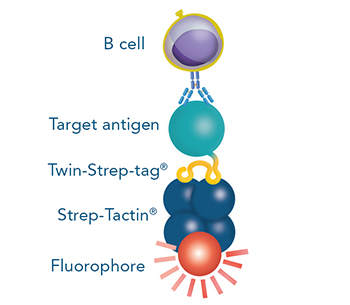
Indirect staining and/or isolation via a protein that is fused to Strep-tag®II or Twin-Strep-tag®, which binds to e.g. a specific receptor on the cell surface.
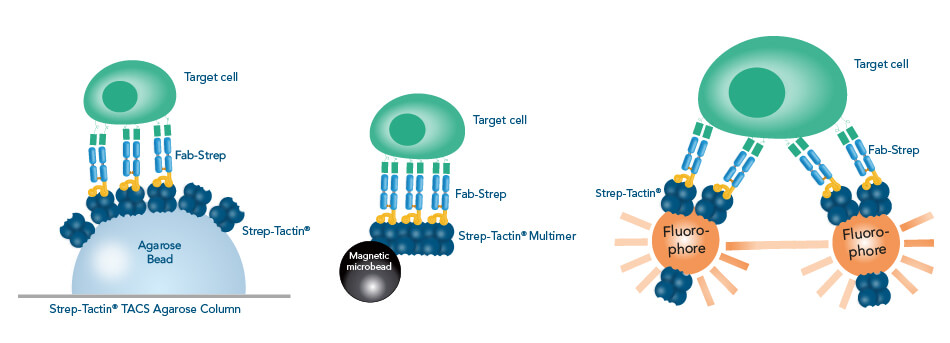
For both ways of selecting cells, different available Strep-Tactin® and Strep-Tactin®XT conjugates offer three basic methods for purifying cells out of mixed samples: Affinity chromatography, magnetic cell isolation or fluorescence activated cell sorting (FACS).
Different conjugates for cell selection
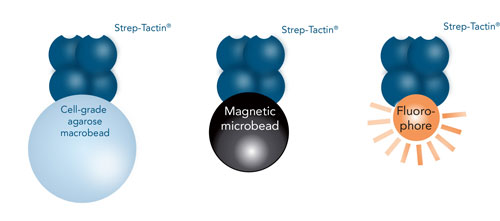
Due to the various available conjugates, the Strep-tag® technology provides a highly flexible and individually adaptable platform not only for proteins, but also for larger molecules such as cells.
Find out more about Strep-tag® cell isolation
Here, we have grouped links to make it easier to find more information about Strep-tag® technology for cell isolation. Find the products that fit your research needs. Have a look at the frequently asked questions about the use of Strep-tag® for cell isolation and references. Find application notes, brochures and flyers in our download area and browse through the application examples.