Unlock the potential of high-affinity purification with Strep-Tactin®XT
Higher protein yields with Strep-Tactin®XT
Differently sized proteins tagged with Strep-tag®II or Twin-Strep-tag® were purified with Strep-Tactin® and Strep-Tactin®XT. In all examples, Strep-Tactin®XT showed the highest binding capacity (1.6-2 fold higher compared to Strep-Tactin®; left graph). In another experiment, a Fab fragment fused to either Twin-Strep-tag® or Strep-tag®II was added to cell lysates at a concentration of 0.6 mg/ml. The amount of purified protein was more than 3-fold lower for the Strep-tag®II-fused Fab fragment if Strep-Tactin® was used (right graph). This could partially be rescued with a Twin-Strep-tag®, which has a higher affinity to both resins. Nevertheless, using Strep-Tactin®XT high capacity resulted in the highest yield independent from affinity tag.
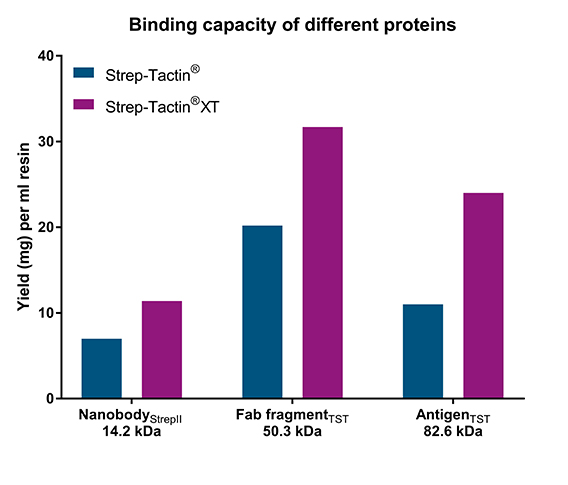
The maximum binding capacities of Strep-Tactin® or Strep-Tactin®XT high capacity was determined with gravity flow columns for three differently sized proteins fused to either Strep-tag®II or Twin-Strep-tag®.
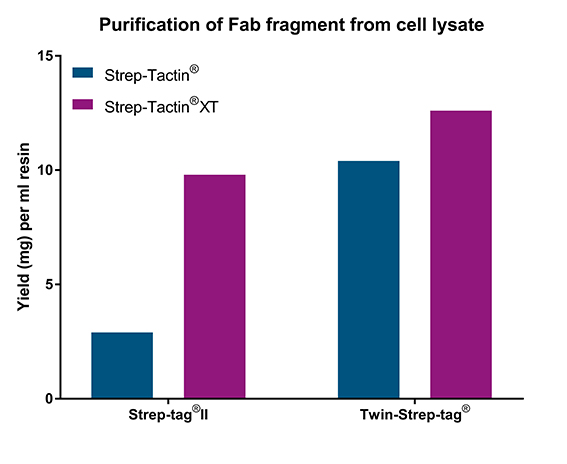
A Fab fragment fused to either Strep-tag®II or Twin-Strep-tag® was added to cell lysates at a concentration of 0.6 mg/ml and subsequently purified using Strep-Tactin® or Strep-Tactin®XT high capacity FPLC columns.
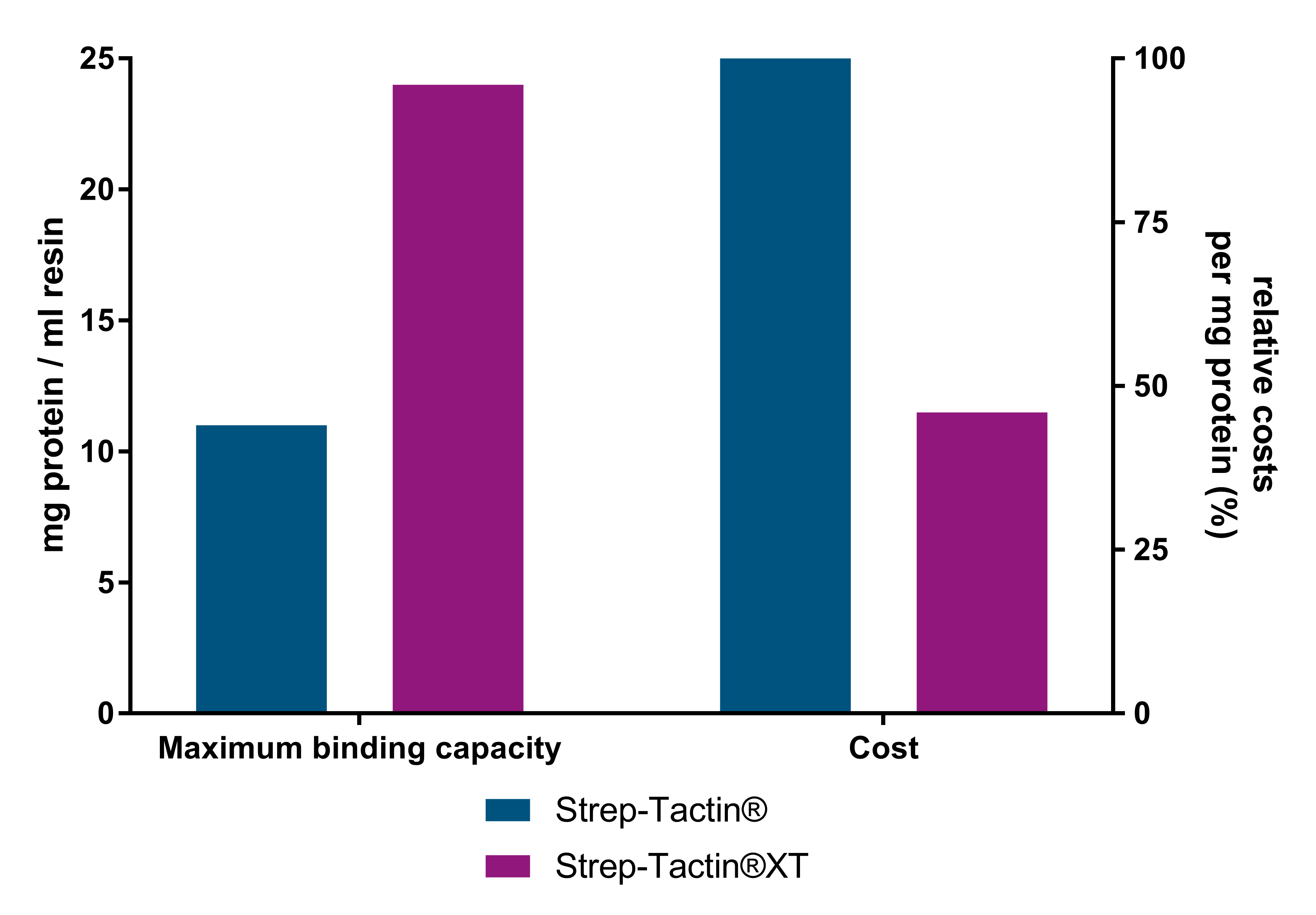
Cost-effective protein purification
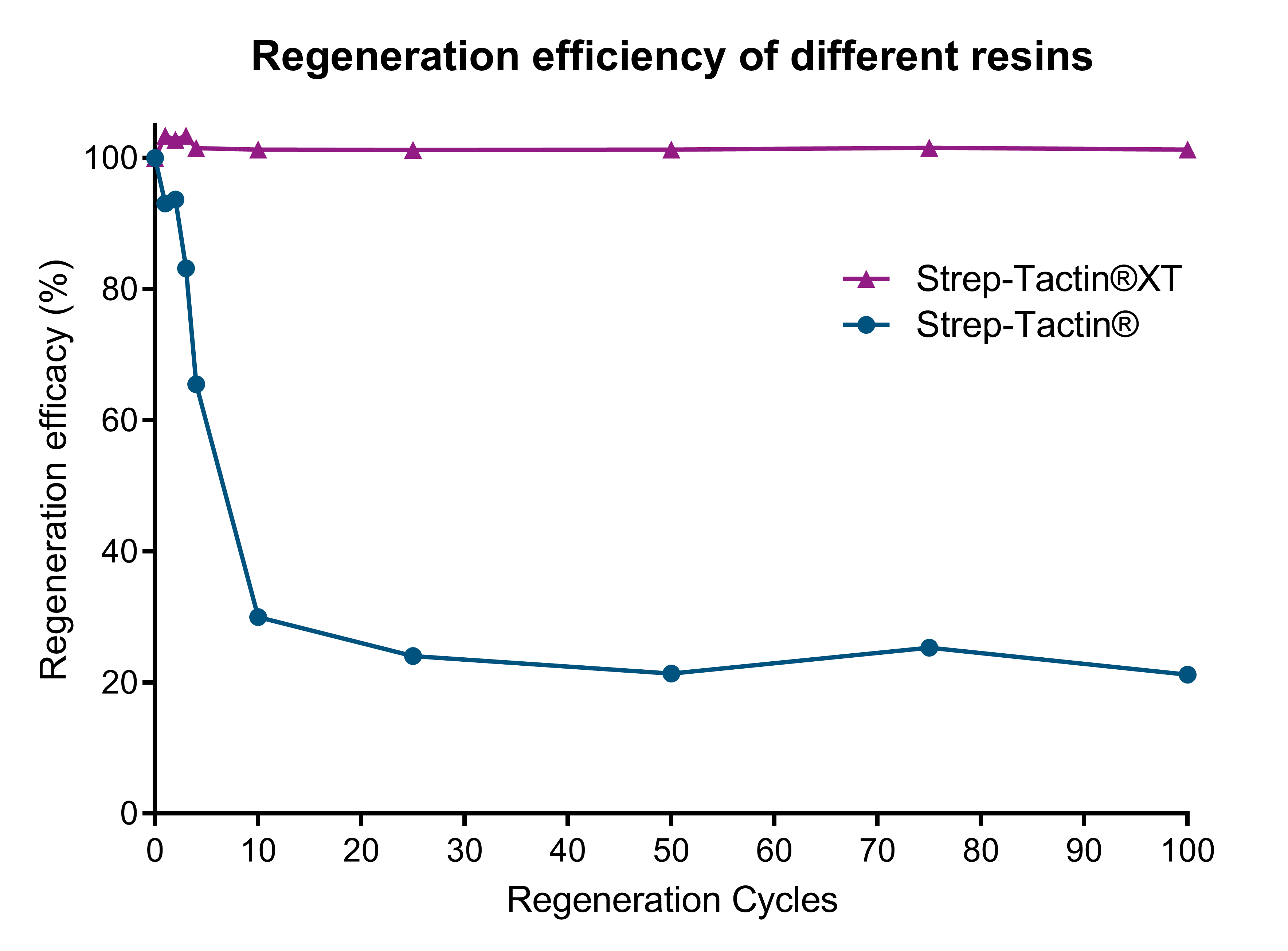
Strep-Tactin®XT resins are the most cost-effective resins due to their higher binding capacity. As compared to Strep-Tactin® resins, Strep-Tactin®XT resin yields more than 2-fold more protein per ml resin and costs up to 54% less per milligram of purified protein. Furthermore, Strep-Tactin®XT prevents the unwanted leakage of the target protein during the washing steps.
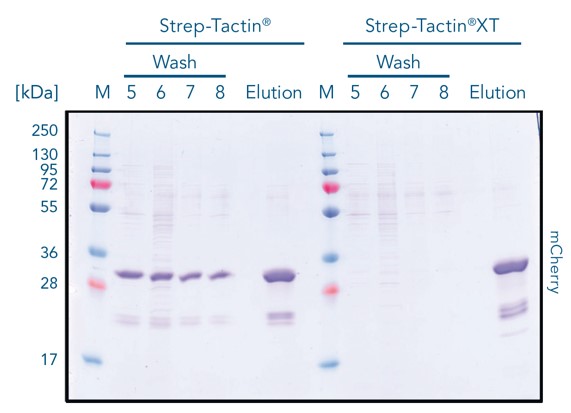
Most efficient binding: Strep-Tactin®XT:Twin-Strep-tag®
Strep-Tactin® and Strep-Tactin®XT were loaded with twin-strep-tagged red fluorescent protein mCherry. Strep-Tactin®XT binds the protein directly in the upper part (right column), while Strep-Tactin® resin binds over nearly the entire resin bed (left column). The lower affinity of the tag to Strep-Tactin® causes the protein to detach more easily, resulting in a lower binding capacity as the protein flows out of the column.

During the washing steps, the protein is tightly bound by Strep-Tactin®XT and no protein is lost during purification. In contrast, significant mCherry loss is observed with Strep-Tactin® resin. This result highlights that Strep-Tactin® resins are not compatible with large samples or washing volumes. In the purification steps, due to the lower affinity, the protein detaches and runs off the column as the column volume is increased.
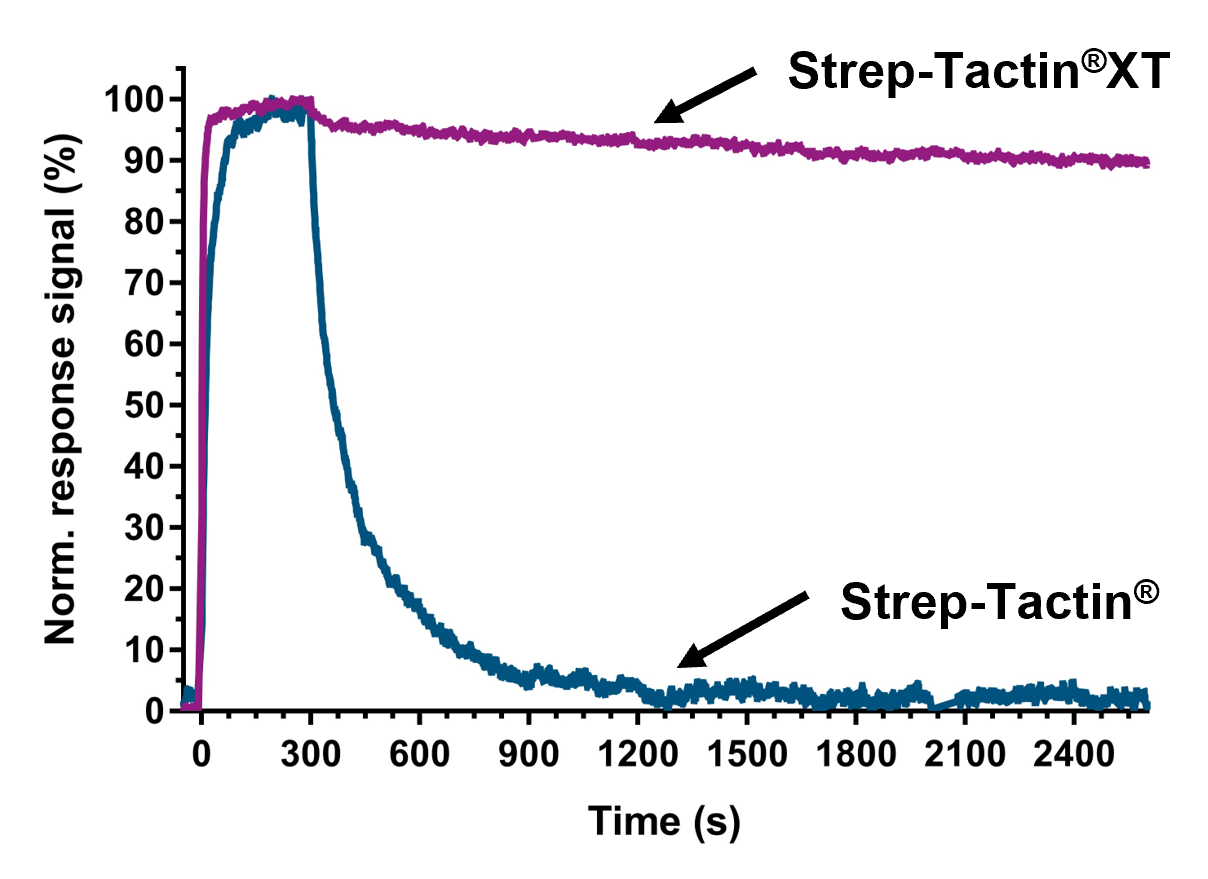
High affinity of Strep-Tactin®XT enables SPR analysis and BLI
Strep-Tactin®XT can be efficiently immobilized on SPR and BLI sensors and enables capturing of TST-fused ligands with exceptionally high affinity in pM range. The system allows kinetic analyses of strong binding analytes with long dissociation times and thus overcomes the current limitations of other affinity tag-based capture systems, such as the His-tag. The possibility to capture diverse ligands even directly from culture media and a simple regeneration procedure of the biosensors add major value to the application of Strep-Tactin®XT in optical biosensor assays.
Read more about Strep-Tactin®XT technology in downstream applications in this application note. Also, find out why Twin-Strep-Tag® in combination with Strep-Tactin®XT is a better alternative to the commonly used Avi-tag in SPR here.
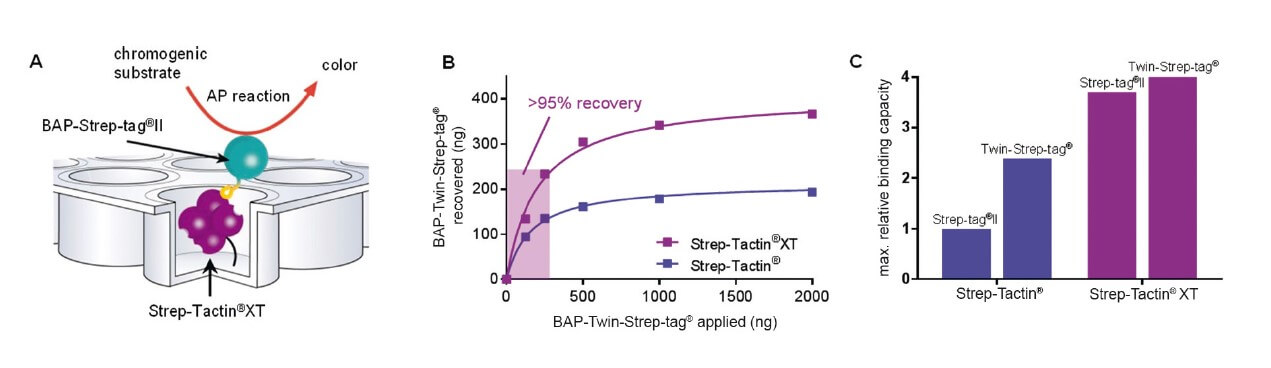
Convenient assays and high-throughput screenings with Strep-Tactin®XT
Strep-Tactin®XT coated microplates ensure convenient assays and high-throughput screenings for strep-tagged biomolecules. Especially, the combination Twin-Strep-tag®:Strep-Tactin®XT is highly stable with a T1/2 of 13 hours and affinity in low pM range. The tag-ligand combination is an efficient and elegant option for an antibody-free immobilization of proteins.
Moreover, the immobilized biomolecules are presented to interaction partners in a uniform manner, which results in reliable and highly reproducible assay formats. This minimizes non-specific binding concomitant with minimal coefficients of variation. Hence, the Strep-Tactin®XT coated microplates are a precise but cost-effective tool for high-throughput screenings and diagnostic assays.
References for Strep-Tactin®XT
Protein purification
- The adaptive landscape of a metallo-enzyme is shaped by environment-dependent epistasis (Anderson, et al. (2021). Nature)
- CryoEM of RUVBL1–RUVBL2–ZNHIT2, a complex that interacts with pre-mRNA-processing-splicing factor 8 (Serna, et. al.(2022). Nucleic Acids Research)
High-throughput purification
- Affinity Purification of TST-Proteins using Strep-Tactin®XT 4Flow® high capacity in IMCStips® (Cook, et al. (2022). Application Note)
SPR
- Application of Strep-Tactin®XT for affinity purification of Twin-Strep-tagged CB2, a G protein-coupled cannabinoid receptor (Yeliseev, et al. (2017). Protein Expression and Purification)
- A conformation-specific nanobody targeting the nicotinamide mononucleotide-activated state of SARM1 (Hou, et al. (2022). Nature Communications)