Monoclonal antibody development & production
The identification and production of monoclonal antibodies is essential for investigating immune responses and developing therapies. In addition, they can be utilized for the direct and indirect detection of antigens in various assays, as capture molecule immobilized on microplates, or as ligand for protein purification, making them a versatile tool in biotechnology.
Different methods were developed for monoclonal antibody discovery including hybridoma technology, phage display or single B cell sorting.
Their common aim is the identification of high affinity antibodies that recognize a specific antigen of choice. This makes the antigen a central component for antibody development. Fusing a Twin-Strep-tag® to an antigen helps to make this central component applicable for various steps in the development process.
The Twin-Strep-tag® suitable for:
- Antigen (protein) purification and analysis
- Immunization
- Antigen-specific B cell staining and isolation via FACS
- ELISA and Western blot
- Biosensor applications
- Biopanning
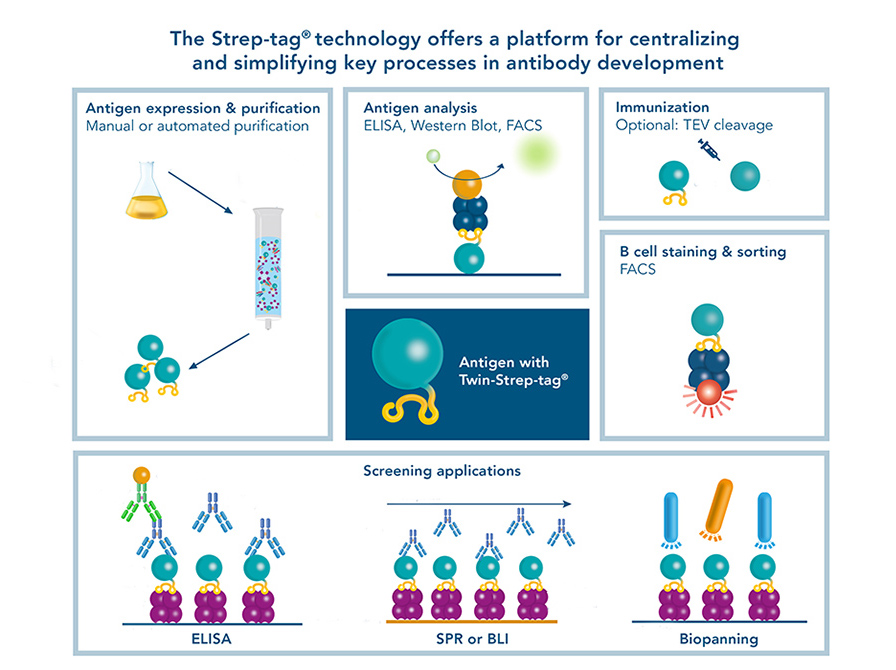
Figure 1: An antigen fused to a Twin-Strep-tag® as central component for all applications needed in antibody development (e.g. with hybridoma technology, single B cell sorting or phage display)
Antigen expression & purification for antibody development
The production of an antigen is a key process in antibody development. It is needed for several steps in the whole procedure such as immunization, screenings and antibody characterizations.
The advantages of using the Strep-tag® system for the antigen purification process are:
- Highly pure antigen preparations suitable for immunization and further downstream processes
- Straightforward isolation procedure without tedious optimization steps
- Reusability even after CIP (Clean-in-place) procedures
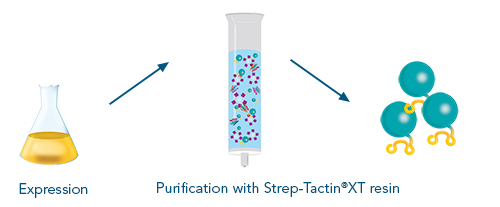
Figure 2: After expressing an antigen with a Twin-Strep-tag®, different formats are available for protein purification. The options range from small to large scale with the possibility to switch between manual and automated systems.
Next to required vectors and an expression system, various purification formats are available, which allow for individual up- or downscaling or automation of the protein production process. In addition to the outstanding target purity, the advantage of fusing an antigen to the Twin-Strep-stag® are the versatile application options for various downstream processes.
The picomolar affinity to its ligand permits the use even for biosensor measurements such as surface plasmon resonance (SPR) or bio-layer interferometry (BLI). This way, switching between different tags depending on the application is unnecessary, which makes the whole procedure quicker and more efficient.
Antigen analysis for antibody development
After the antigen was purified from sources such as cell lysates or cell culture medium, the purity, integrity and concentration should be determined. Coomassie or silver staining as well as analytical size exclusion chromatography are options for determining if unspecific proteins are in a sample. Detecting the produced antigen via its Twin-Strep-tag® in a Western blot is a way to identify if the protein was produced successfully at the expected size and no aggregation or degradation products were formed. To determine the concentration of the yielded antigen, ELISA is a suitable method.
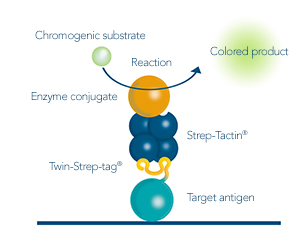
Figure 3: Different Strep-Tactin® conjugates allow for analysis methods such as western blot or ELISA.
Immunization for antibody development
The purification of proteins with the Strep-tag® technology results in a highly pure antigen that is suitable for immunizing a host species. Additional optional processing steps include the removal of biotin, the eluent, from the elution fraction via dialysis or size exclusion and/or TEV cleavage of the Twin-Strep-tag®. However, since the tag has a small size of only 28 amino acids, the immunogenicity is expected to be low.
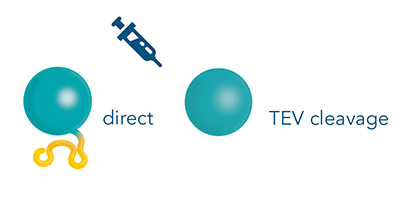
Figure 4: The Twin-Strep-tag® is a short peptide, therefore the removal prior to immunization is not strictly necessary. Optionally, the tag can be removed via a TEV protease.
Antigen-specific B cell staining & sorting for antibody development
For some antibody development approaches, antigen-specific B cells are isolated from blood or other tissues of seropositive individuals, which encountered the antigen by infection or immunization. Here one can make use of the antigen fused to the Twin-Strep-tag® by incubating it with:
- Fluorescent Strep-Tactin® or Strep-Tactin®XT conjugates or
- Strep-Tactin® Magnetic Microbeads or
- Strep-Tactin® TACS Agarose
This generates selection reagents that are suitable for the staining and sorting of antigen-specific B cells via flow cytometry, magnetic or affinity chromatographic isolation, respectively.
B cells that recognize the antigen of choice are selected from e.g. peripheral blood mononuclear cells (PBMCs). Single cells can be sorted directly into lysis buffer for antibody sequencing or taken into culture for antibody production. This method for monoclonal antibody production offers the possibility to extract B cells and consequently also antibody sequences directly from humans, thereby enhancing the chance to identify antibodies that are readily suitable for therapeutic purposes.
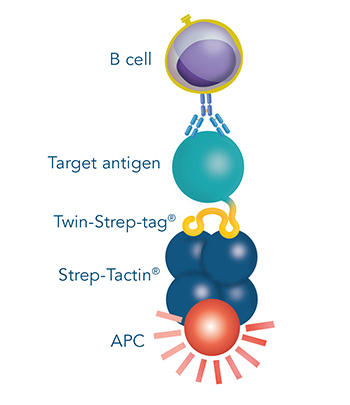
Figure 5: Via its tag, the antigen can be combined with Strep-Tactin® conjugates for e.g. fluorescent staining and sorting of B cells.
Example publications for antigen-specific B cell staining & sorting
Clone/antibody screening & antibody characterization for antibody development
Independent from the method chosen for monoclonal antibody discovery, screening steps are key to finally identify the best performing antibody.
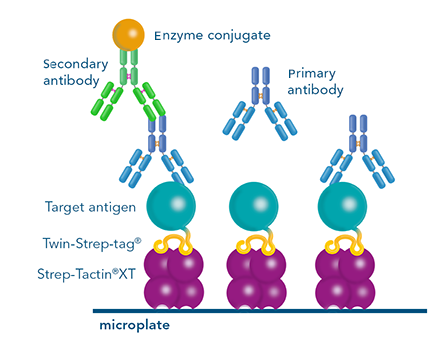
Figure 6: An antigen can be immobilized on Strep-Tactin®XT coated microplates via its Twin-Strep-tag® for antibody screening with ELISA. Due to the reusability of the plates, several screening rounds are possible.
ELISA
A Twin-Strep-tag® - antigen can be used for antibody screening by ELISA. Via its tag, it can be immobilized on Strep-Tactin®XT coated microplates. This way, the microplate is functionalized for capturing antibodies from e.g. B cell supernatants. Subsequent detection indicates which B cell clones produce specific antibodies. Usually, several selection rounds are required to find the best performing clone. Since Strep-Tactin®XT coated microplates can be regenerated several times, using the Strep-tag® technology in this process is cost saving and time efficient.
Biopanning
Monoclonal antibody discovery by phage display uses a library of antibody fragments that are expressed on the surface of bacteriophages. To find those bacteriophages that present antibody fragments that detect the antigen, a selection procedure called biopanning is utilized. For this method, the phages are exposed to an immobilized antigen. The ones that bind are eluted, amplified and used in a next selection round. For the selection rounds, Strep-Tactin®XT coated microplates with an immobilized Twin-Strep-tag® antigen offer a reusable, cost-saving system similar to antibody screening by ELISA. After the identification of antibody fragments that specifically bind the antigen, sequences have to be cloned into full length antibodies for further use in research or therapeutic applications. Since this step can potentially change the features of an antibody, additional characterization steps are necessary, making this method for monoclonal antibody discovery overall very labor-intensive.
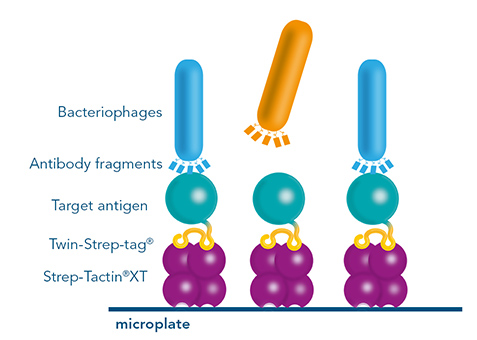
Figure 7: Strep-Tactin®XT coated microplates are suitable for biopanning, a selection procedure employed in phage display. The plates can be regenerated several times, therefore multiple selection rounds are possible.
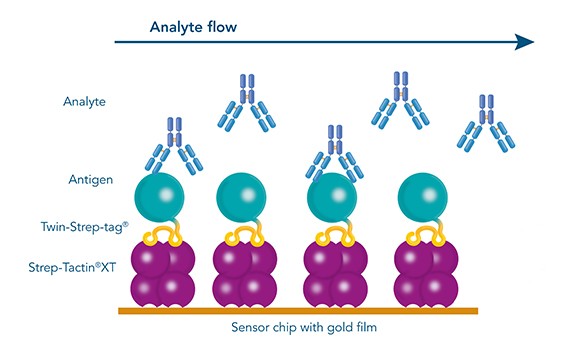
Figure 8: Due to the picomolar affinity of Strep-Tactin®XT to the Twin-Strep-tag®, biosensor applications such as surface plasmon resonance (SPR) are possible.
SPR & BLI
The antibodies that are yielded by different antibody discovery and development approaches can be further characterized by biosensor applications such as surface plasmon resonance (SPR) or Bio-layer interferometry (BLI). Again, the Twin-Strep-tagged antigen is central in those analyses. Due to the picomolar affinity of the Twin-Strep-tag® to Strep-Tactin®XT, the antigen is immobilized sufficiently to allow the determination of binding kinetics to a selected antibody. This way, antibodies with the desired affinities are identified and chosen for large scale productions.
Antibody purification
After the best antibody was identified for a specific antigen, it has to be expressed in e.g. a cell culture system. The produced antibodies subsequently have to be extracted from cell lysates, cell culture supernatants, or biological probes. The purification can take place without affinity tag via the physicochemical properties, antigen-specific affinity or the antibody class. Physicochemical properties are the molecular weight, charge, or clusters of specific residues.
Depending on the origin of the sample, purification with the help of physicochemical properties or antigen-specific affinity can lead to the isolation of further proteins and antibodies with similar properties besides the target antibody. Thus, if a specific antibody with a high purity should be obtained, antibody class-specific affinity chromatography is recommended, such as Protein A, which can bind immunoglobulins (antibodies) within the Fc region of their heavy chain without regard to antigen specificity.
Choosing an affinity tag-based purification procedure is also possible and can be beneficial, especially if the antibody is to be implemented in subsequent immobilization or detection. For this purpose, the application of the Strep-tag® technology is highly recommended. The antibody can simply be isolated in high purity with a resin perfectly suitable for large proteins, Strep-Tactin®XT 4Flow®. The different formats available for protein purification make it a very flexible system that allows for individual adaptations of sample number and size.

